TBCK: TBC1 Domain Containing Kinase
This gene encodes a protein that contains a protein kinase domain, a Rhodanase-like domain and the
Tre-2/Bub2/Cdc16(TBC) domain. The encoded protein is thought to play a role in actin organization, cell growth and cell proliferation by regulating the mammalian target of the rapamycin (mTOR) signaling pathway. This protein may also be involved in the transcriptional regulation of the components of the mTOR complex. Alternative splicing results in multiple transcript variants.
https://www.ncbi.nlm.nih.gov/gene/93627
What is the TBC Domain?
- Evolutionarily conserved protein domain
What is a Kinase?
Catalyzes transfers of phosphates from high energy phosphate-donating molecules to specific substrates. E.g., ATP -> ADP, something that happens 4 times during glycolysis. The production of energy during glycolysis is exactly that process of ATP -> ADP, as discussed in THE PROOF episode with Kieron Rooney.
Protein Kinase
A Kinase which acts strictly on proteins as opposed to lipids, carbs, or other molecules.
Ribosomes, Mitochondria and Energy (ATP)
You can only turn fat into two carbon subunits that get put into the mitochondria where you very efficiently make ATP, carbon dioxide, and water vapor*.
Peter Attia from Rich Roll 695 (citation needed)
Glycolysis isn’t less efficient than fat, both pyruvate and fatty acids as the input into the mitochondria are very efficient, the problem actually is that there isn’t enough oxygen to oxidize the pyruvate, so the pyruvate gets turned into lactate, which yields 1/16th the ATP.
What is the mTOR Pathway?
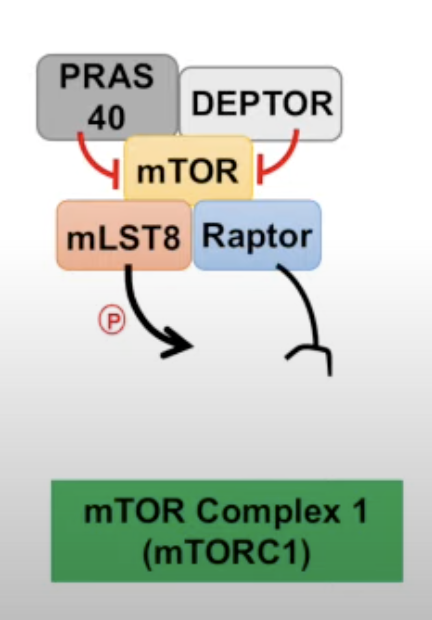
- Complex 1 (mTORC1)
- mTOR and Raptor
- mLST8 (\(G \beta L\)), stabilizes the kinase loop. It allows mTOR to phosphorlate its target.
- PRAS40 and DEPTOR inhibitors
- Complex 2 (mTORC2)
- DEPTOR inhibitor
- Protor1/2 and mSin1 are regulators
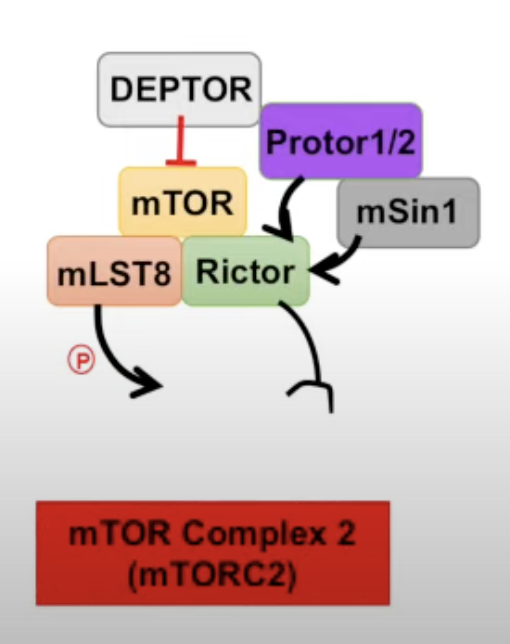
Drugs are used to inhibit the mTOR pathway, one of which is rapamycin. Apparently rapamycin is only useful in complex 1.
Autophagy
Autophagy is lysosomal recycling, essentially. It works via phagophore which engulfs intracellular “cargo”, such as protein aggregates, ribosomes, and organelles. This phagophore then fuses with the lysosome, where its cargo is degraded via the lysosome’s acid proteases. The byproducts of degradation are sent back out to the cytoplasm where they can be reused for metabolism. This includes ATP generation.
Lysosome
The lysosome is a cell organelle that contains digestive parts. It breaks down excess or worn out cell parts, they may also be used to destroy invaders. Additionally, lysosomes may play a role in self-destruction / programmed cell death / apoptosis.
Storage Diseases
The TBCK paper from 2017 mentions similar “coarse facial features” to those individuals affected by storage diseases. A storage disease is a lysosomal disease, wherein the lysosome fails to do its autophagic work, leaving the cell overloaded with substrates.
The Logic of Protein
These stream diagrams or whatever they’re called are interesting. The red T arrow indicates an inhibitory relationship while the black arrow indicates activation; but, as these things compose, their relationships follow a logical dual. Here’s a few examples:
- \(A \dashv B \to C\): A inhibits B, which activates C, so A inhibits B
- \(A \dashv B \dashv C\): A inhibits B, which inhibits C, so A activates C.
It’s the composition of two inhibitory relations that produce an activating one. And the same for the composition of two activating relations. Sounds like this is only the case for inhibitory relations.
Leucine
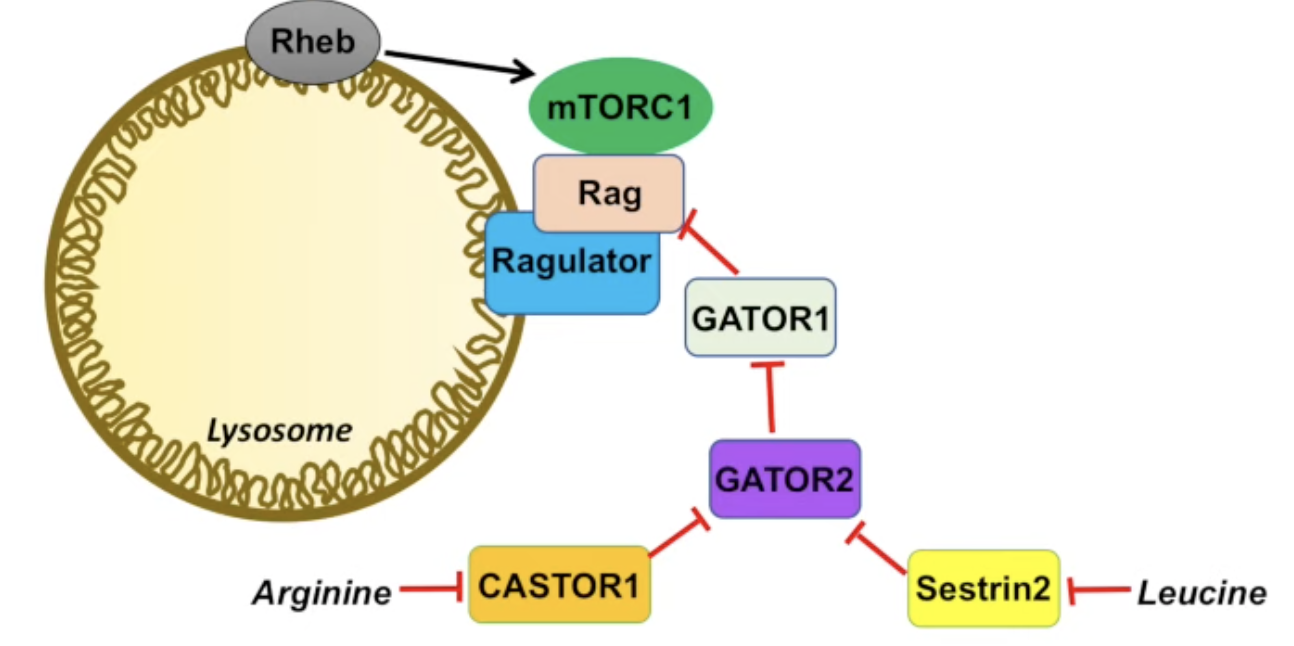
Following the logic here—that the composition of two inhibitors make an activator—we can see that leucine is an activator for mTORC1:
\[ \text{Leucine} \dashv \text{Sestrin2} \dashv \text{GATOR2} \Rightarrow \text{Leucine} \rightarrow \text{GATOR2} \\ \text{GATOR2} \dashv \text{GATOR1} \dashv \text{RAG} \Rightarrow \text{GATOR2} \rightarrow \text{RAG} \]
Therefore:
\[ \text{Leucine} \rightarrow \text{Rag}\ (\text{mTORC1}) \]
TBCK has been shown to regulate the mammalian target of rapamycin (mTOR) signaling pathway, which is also stimulated by exogenous leucine supplementation. TBCK was absent in cells from affected individuals, and decreased phosphorylation of phospho-ribosomal protein S6 was also observed, a finding suggestive of downregulation of mTOR signaling.
This is from the abstract of Mutations in TBCK, Encoding TBC1-Domain-Containing Kinase, Lead to a Recognizable Syndrome of Intellectual Disability and Hypotonia (emphasis is mine, though).
It feels like this implies that TBCK and leucine both have the same job—activating mTORC1, so supplementing with leucine could “make up for” the missing or pathologically low levels of TBCK. So the hypothesis is correct here, but the causality is not:
knockdown of TBCK decreases the transcription of mTOR complex (mTORC) proteins and downregulates mTOR signaling.
This is why leucine helps, though, as it activates mTORC1.
phospho-ribosomal protein S6, and its phosphorylation, and what
happens to S6 when it isn’t getting phosphorylated. Lysosome stress?
Storage disease?
No, rereading this, it sounds like decreased phosphorylation of S6 is actually a sign that there’s been down regulation along mTOR.